PBS: the Plasma Bottle Sampling®
Ensuring the traceability of plasma and delivering transparent information
Technical file
Type of innovation: System
Scope: Bioengineering
Innovation leader: Shared leadership
Year: 2009
Period: From 2003
Geographical scope: International
Economic impact: High
Level of innovation: Disruptive
Patent: Yes
Interdisciplinary connections: -
The manufacture of plasma products that meet the highest safety and quality standards is a long and complex process, one that starts with donation of the plasma and goes all the way through to the final use of the resultant medicines to help patients. To guarantee the safety of the end product, every unit of donated plasma has to be traceable at each stage of the donation and manufacturing process so that it can be linked to the corresponding batches of the finished product. However, it is the first stage of the process – donation – which poses the greatest challenge to the need for error-free traceability because the manual processing of samples raises the possibility of mislabeling at the outset. In response to this challenge, Grifols decided to design an automatic identification process.
Automating the preparation of plasma samples
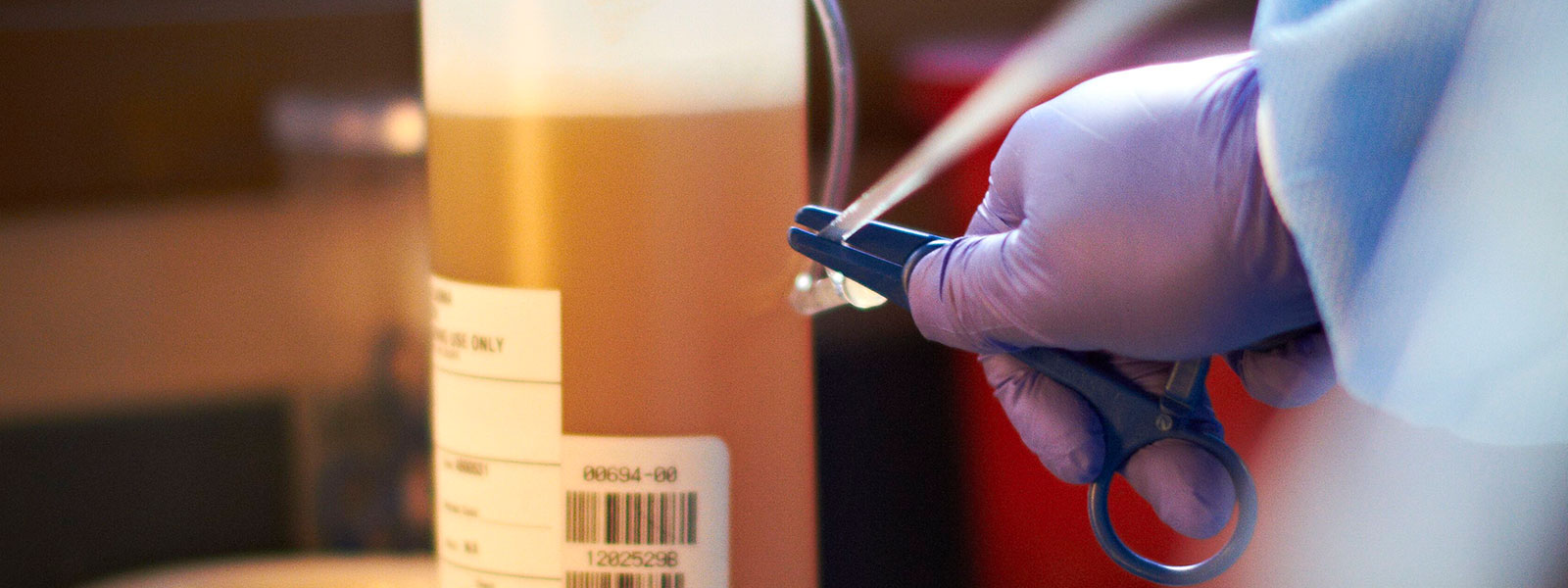
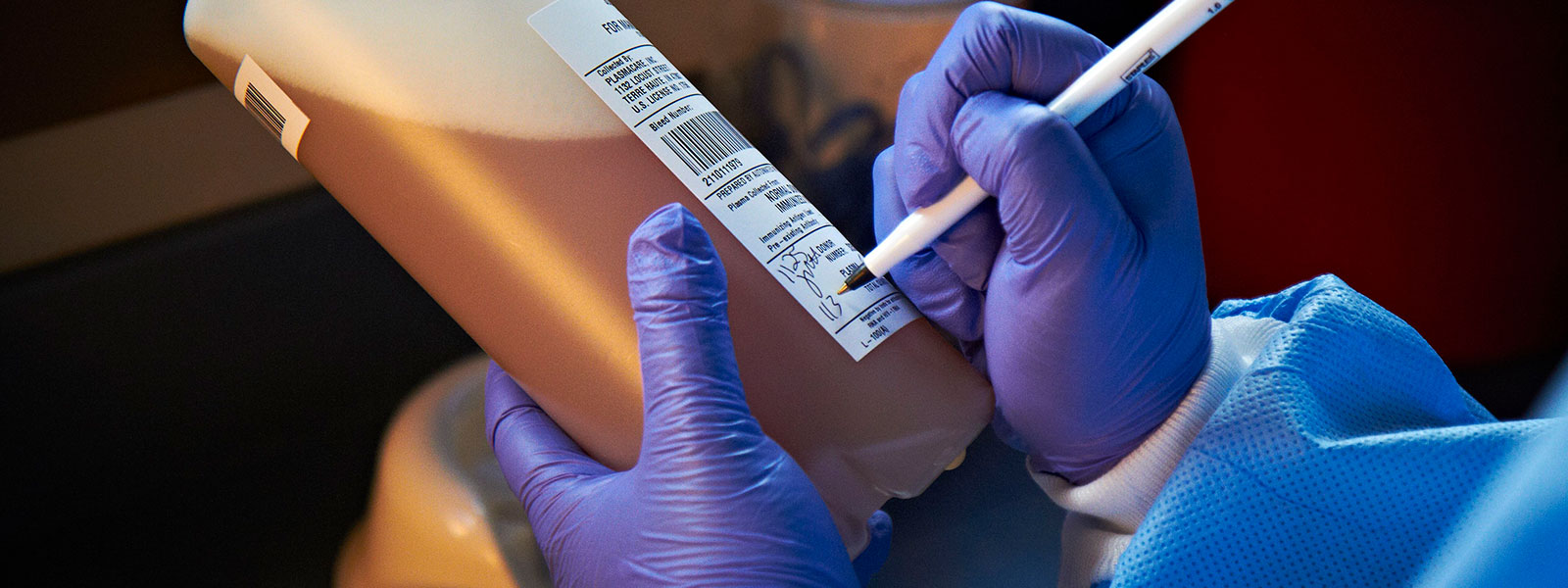
In 2009, Grifols designed the Plasma Bottle Sampling® system or PBS®. This innovative solution was developed by the applied engineering department of Grifols Engineering, and automated the preparation of samples for analysis. Each unit of donated plasma consists of approximately 830 mL of plasma in a polyethylene container. The system uses a code reader to automatically identify the plasma unit, and then dispenses a small volume of plasma into the sample tubes for subsequent laboratory testing. The tubes are coded and labeled to ensure the samples are correctly matched with the plasma unit.
The first version of this system was tricky to operate and required a lot of maintenance. However, it was soon replaced by a smaller, simpler version, the PBS® Little. Although this solution was originally developed for use in the Grifols network of donor centers, its success led to it being sold to third parties.

Software compatibility
The PBS® system enables data to be shared with PediGri®, Grifols software which provides detailed information about each plasma unit, ensuring full traceability at every stage, and offering greater information transparency for health professionals, who have access to all the specific information about the entire process associated with the individual vial they are administering to their patients.
“The system uses a code reader to automatically identify the plasma unit, and then dispenses a small volume of plasma into the sample tubes for subsequent laboratory testing. The tubes are coded and labeled to ensure the samples are correctly matched with the plasma unit.”
Bibliography
López Alvárez, D., Roura Adell, S., Rodríguez García, F., Causi Casamor, O. y Wada, S. (2009). Dispositivo para la extracción automática de muestras de líquido de recipientes colectores y procedimiento para realizar dicha extracción. (Oficina Española de Patentes y Marcas Patente Nº ES20090000138). Link.
Grifols, S.A. (2012, october). El nuevo sistema Plasma Bottle Sampling automatiza la preparación de muestras para análisis. [Press release].
Roura Fernández, C., García Sánchez, M. y Fleta Coit, D. (2014). Dispositivo de toma de muestras de contenedores. (Oficina Española de Patentes y Marcas Patente Nº ES20140030162). Link.