Adverse reactions - Pharmacovigilance
Pharmacovigilance is the science and activities relating to the detection, assessment, understanding and prevention of adverse reactions and other medicine-related problems. Medicines may affect the body in unintended, harmful ways. These effects, called side effects or adverse reactions, represent risks of medicines. It is therefore important to identify any new or changing risk of a medicine as quickly as possible, and to take measures to minimize risk and promote safe and effective use.
Please fill out the following form if you think you could have presented any side effect or adverse reaction after receiving treatment with a Grifols product.
This form is also available in other languages. See the form in Arabic or French.
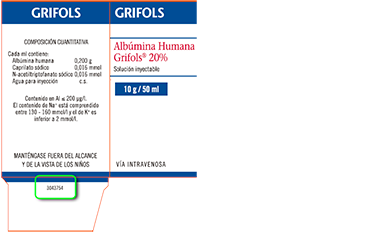
?Where to find the SAP code: