Chronic liver disease is an all too common and growing problem in the developed world, with worldwide prevalence rates of around 20%.1 One such condition is cirrhosis, in which the liver is permanently scarred and in many cases can lead to liver failure.2 Grifols recognizes the huge potential for plasma-derived products to treat severe liver diseases.
Albumin and liver disease
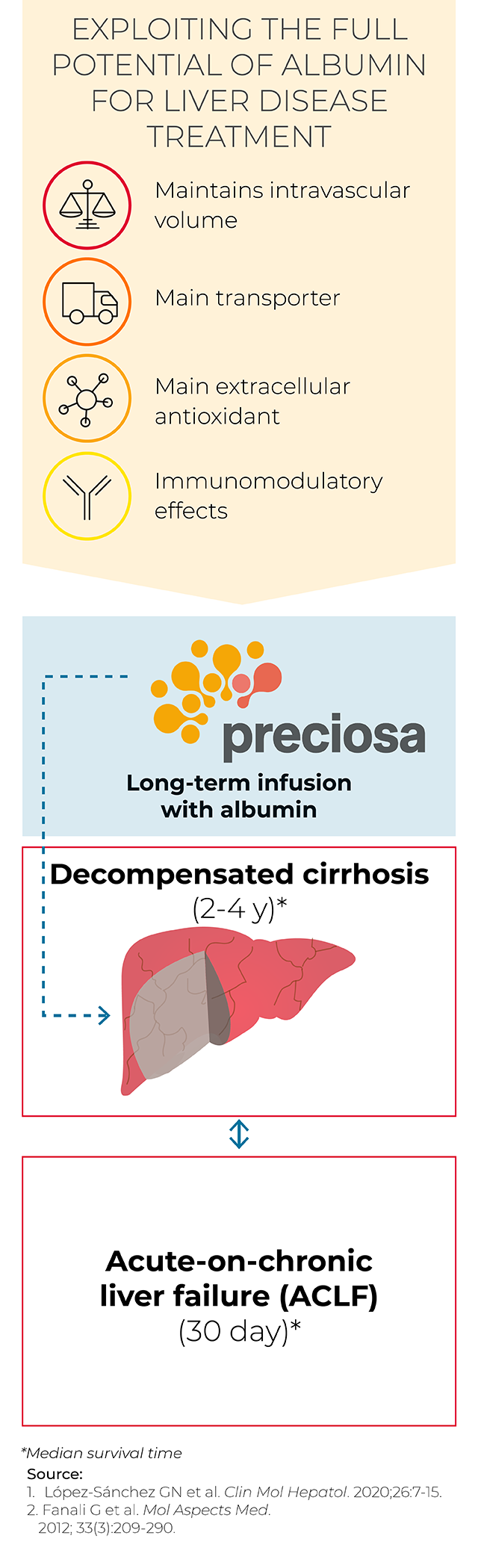
Our increasing understanding of the multifactorial mechanism of action of albumin, the most abundant protein in plasma, provides vast potential to treat liver disease.
Taking into account the hemodynamics and systemic effects of albumin treatment, including the antioxidant and anti-inflammatory properties of this protein and its role in metabolite transportation,3-4 we’re using albumin therapies to manage different stages of cirrhosis and circumvent complications (including organ failure and systemic inflammation).

PRECIOSA trial
Phase 3 international study evaluating the use of continuous albumin administration in reverting cirrhosis progression.5 We have demonstrated that albumin dysfunction correlates with disease markers of cirrhosis,6 and recent clinical data have already shown an increase in albumin levels along with a reduction in systemic inflammation in patients with decompensated cirrhosis following albumin treatment.7
Learn about our latest approved albumin portfolio innovation, a convenient, flexible easy-to-use albumin bag, here.
1 Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650-2666. doi: 10.1016/j.cgh.2019.07.060
2 López-Sánchez GN, Dóminguez-Pérez M, Uribe M, Nuño-Lámbarri N. The fibrogenic process and the unleashing of acute-on-chronic liver failure. Clin Mol Hepatol. 2020;26(1):7-15. doi: 10.3350/cmh.2019.0011
3 Duran-Güell M, Flores-Costa R, Casulleras M, et al. Albumin protects the liver from tumor necrosis factor α-induced immunopathology. FASEB J. 2021;35(2):e21365. doi:10.1096/fj.202001615RRR
4 Fanali G, di Masi A, Trezza V, Marino M, Fasano M, Ascenzi P. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33(3):209-90. doi: 10.1016/j.mam.2011.12.002
5 Effects of long-term administration of human albumin in subjects with decompensated cirrhosis and ascites (PRECIOSA). ClinicalTrials.gov identifier: NCT03451292. Accessed April 27, 2022. https://www.clinicaltrials.gov/ct2/show/NCT03451292?term=preciosa+albumin&cond=cirrhosis&draw=2&rank=1
6 Horrillo R, Mestre A, Ortiz AM, et al. Albumin dysfunction correlates with disease markers in patients with decompensated cirrhosis. Poster presented at: Virtual International Liver Conference™ 2021; June 23-26, 2021. Accessed April 28, 2022.
7 Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. doi: 10.1053/j.gastro.2019.03.021
Developing Treatments
Patients are at the center of our far-ranging scientific research to develop therapeutic solutions to some of the world’s most pressing healthcare challenges.