The Griflow® device
An invention to regulate the flow of intravenous infusions
Technical file
Type of innovation: Instrument; Procedure
Scope: Hospital Pharmacy
Innovation leader: Grifols i Lucas, Víctor
Year: 1998
Period: 1972-2002
Geographical scope: International
Economic impact: Low
Level of innovation: Disruptive
Patent: Yes
Interdisciplinary connections: -
Griflow® was a medical device that regulated the flow rate for catheters and for infusion systems in general. The device included a PVC tube that was incorporated into the infusion kit. Heat was applied to this to produce a capillary of the required section and length, to allow the desired flow of the liquid, and with an internal regulator to replace the conventional infusion clamp to control the drip rate.
Both the diameter and the length of the tube could be accurately controlled, and the tube could be inserted into a perfusion kit to deliver a constant flow. It was very simple to operate but a major drawback was that it did not allow the dose to be changed. Despite this, it was potentially very useful in cases where an additional route to the one being used to administer a drug was required.
The lack of variable regulation represented a major barrier when it came to marketing the device. However, an alternative application was found for the device, as a means of limiting maximum flow. In conventional devices, if the clamp is accidentally opened too wide, the increased flow can endanger the patient. Griflow® would thus act as a safety measure by setting a limit on maximum flow.
“Both the diameter and the length of the tube could be accurately controlled, and the tube could be inserted into a perfusion kit to deliver a constant flow.”
Two tubes are better than one
Griflow® Dual was developed five years after Griflow®. It was designed for the infusion of Flebogamma®, a gammaglobulin product that could provoke an adverse reaction in the patient during the infusion of the first milliliters into the blood. Griflow® Dual used two separate tubes to enable the flow of medicinal products to be regulated at two speeds: a slower rate at the start of infusion, and a higher rate if the medicine was well tolerated by the patient. The option of two-speed infusion improved patient safety and made the administration process more efficient.
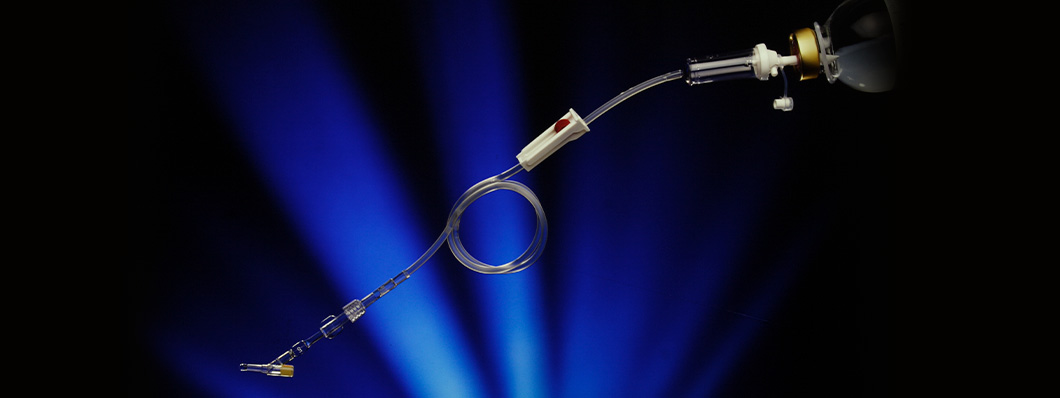
Market barriers
Although both systems were tested in several hospitals, neither of them was ever released commercially, both for technical reasons and due to cost considerations. The technical problem related to the fact that, depending on the size and position of the infusion kit (length of tube and height of column, which was adjusted according to the patient's position), the pressure to the device changed and could cause reduced accuracy and unreliable operation. An added problem was the fact that the flow could not be regulated. At the same time, the cost was high in comparison to other infusion components. The large quantities of infusion equipment used by hospitals meant that the additional cost of the device was too high to be justified in terms of added value, and made successful marketing of the device impossible.
Bibliography
Grifols i Lucas, V. (1991). Regulador de caudal para catéteres y similares y procedimiento para la fabricación del mismo. (Oficina Española de Patentes y Marcas Patente Nº ES2028740 (A6)). Link
Grifols i Lucas, V. (2001). Dispositivo y procedimiento para el control de caudal para productos medicamentosos. (Oficina Española de Patentes y Marcas Patente Nº 200100472). Link
Grifols, S.A. (2011). ¿Y si lo hacemos así? Patentes de Víctor Grífols i Lucas. Barcelona: Grifols, S.A.
Related innovations
INSTRUMENT
Infusion equipment