November 5, 2020
Grifols continues delivering solid revenue and earnings growth driven by the Bioscience and Diagnostic Divisions
-
Grifols’ revenues reach EUR 4,031 million and grow by 7.8% (7.6% cc1 driven by the Bioscience Division, which increases by 10.1% (9.8% cc) to EUR 3,242 million, and the Diagnostic Division, which grows by 2.3% (2.5% cc) to EUR 546 million
-
Both divisions report significant growth in the third quarter: Bioscience reports EUR 1,084 million in revenues (5.7%; 9.0% cc) and Diagnostic, EUR 206 million (11.2%; 14.0% cc)
-
In the third quarter, EBITDA margin reaches 29.8% to EUR 404 million, marking the Grifols’ highest-ever quarterly figure. EBITDA for the first nine months totals EUR 984 million. This is the result of all efforts made across the board and specially on innovation, as well as the ongoing operational containment plan in these unprecedented times
-
Net profit increases by 14.7% to EUR 486 million
-
Grifols closes the acquisition of a fractionation plant and two purification plants in Canada, as well as 11 plasma centers in the U.S.
-
Grifols reaches an agreement to acquire the remaining equity of Alkahest, in line with its strategic focus on innovation and R+D on leading-edge therapies
-
Grifols underlines the vital role of plasma and the need to increase plasma donations during the 2020 International Plasma Awareness Week, organized by the Plasma Protein Therapeutics Association (PPTA)
Barcelona, November 5, 2020.- Grifols (MCE: GRF, MCE: GRF.P, NASDAQ: GRFS) continues to demonstrate its resilience and commitment to sustainable growth and innovation amid the current COVID-19 pandemic. Profitability and the favorable evolution of margins remain core priorities.
Raimon Grífols and Víctor Grífols Deu, Co-CEO’s state that “as in previous quarters, we want to acknowledge our team for their perseverance, dedication and commitment with Society, which ensure Grifols’ life-saving therapies, products and services reach our patients. We also remain resolute in our fight against COVID-19 and deeply confident in the potential of plasma and its derived medicines to combat the SARS-CoV-2 virus.
Today, more than ever, we want to appeal to the generosity and commitment of all our donors, without whom the availability of plasma-derived medicines would not be possible. For this reason, we join the Plasma Protein Therapeutics Association’s plea, made during the International Plasma Awareness Week, urging European authorities to take decisive action to increase plasma donations.
We continue committed to all of our key stakeholders and this third quarter 2020 results are a clear delivery of this commitment. In particular, the company’s revenue growth and operating profitability are a good indication of our value creation driving strategic investments and innovation, while remaining faithful to the same highest-social commitment and ethics that have guided us throughout our history”.
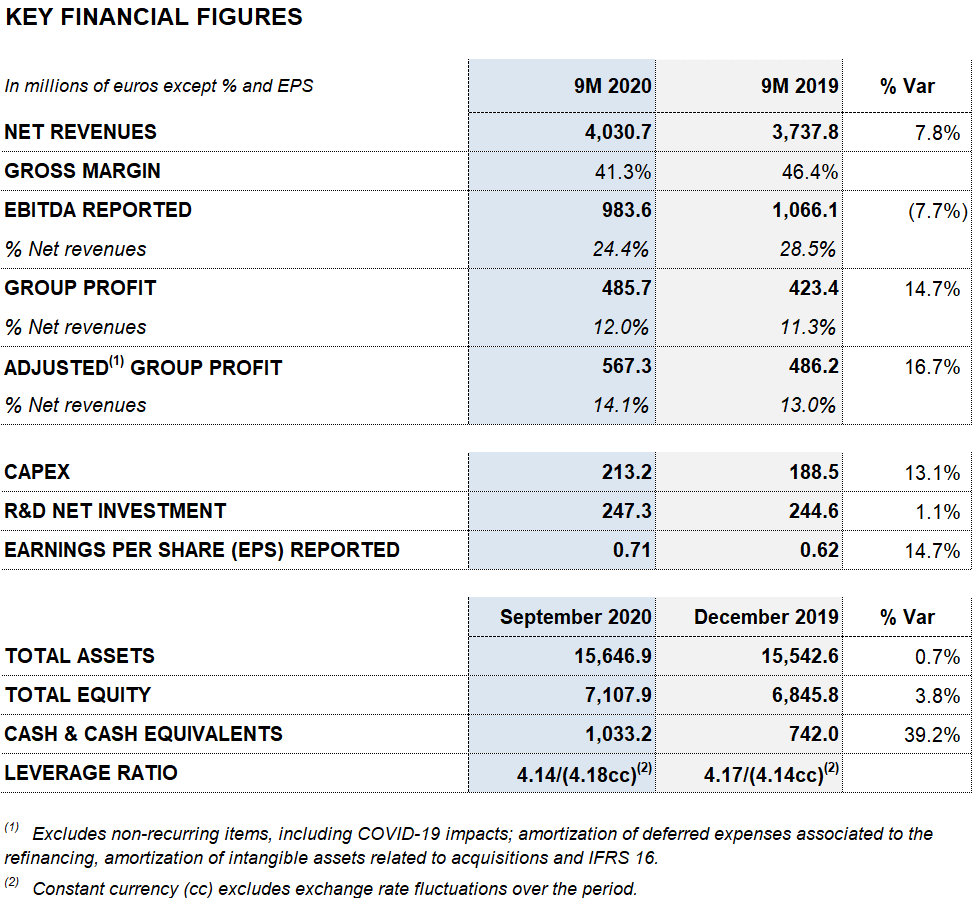
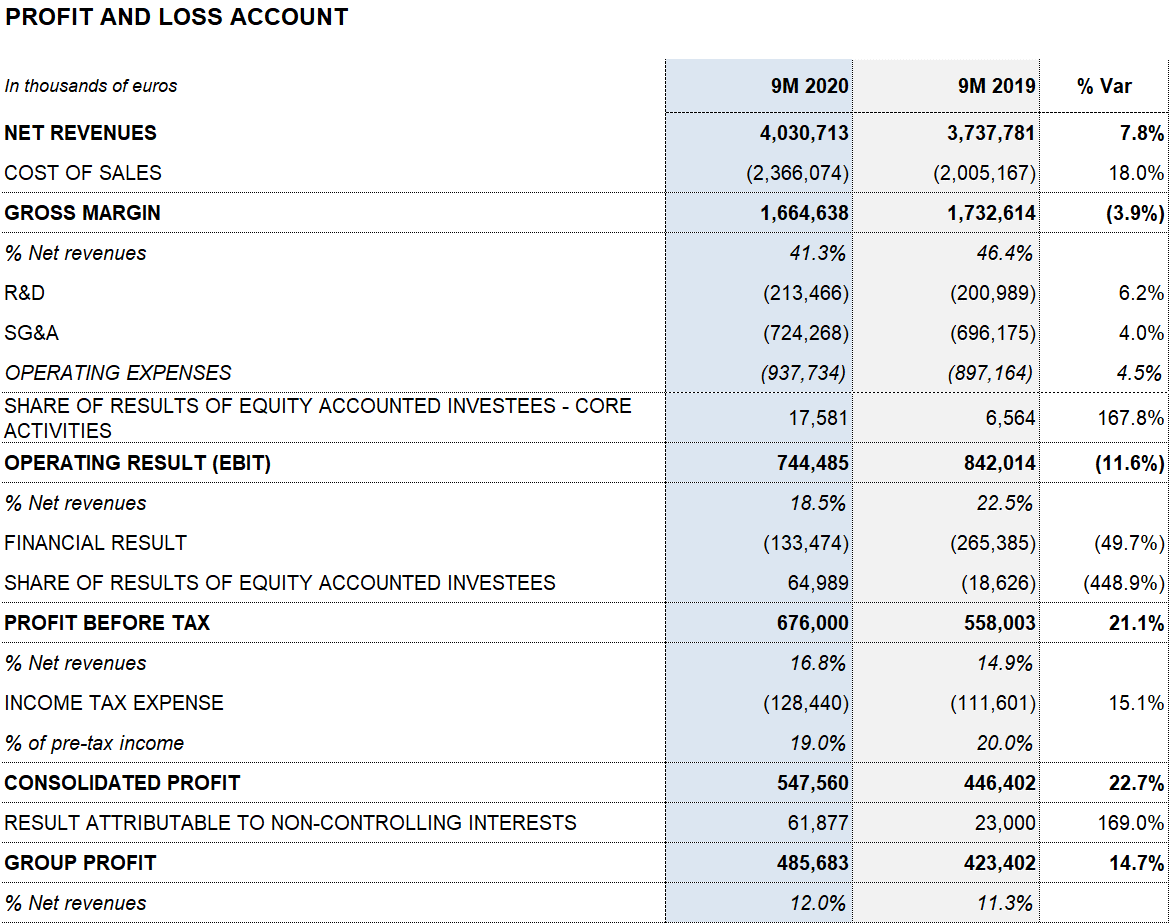
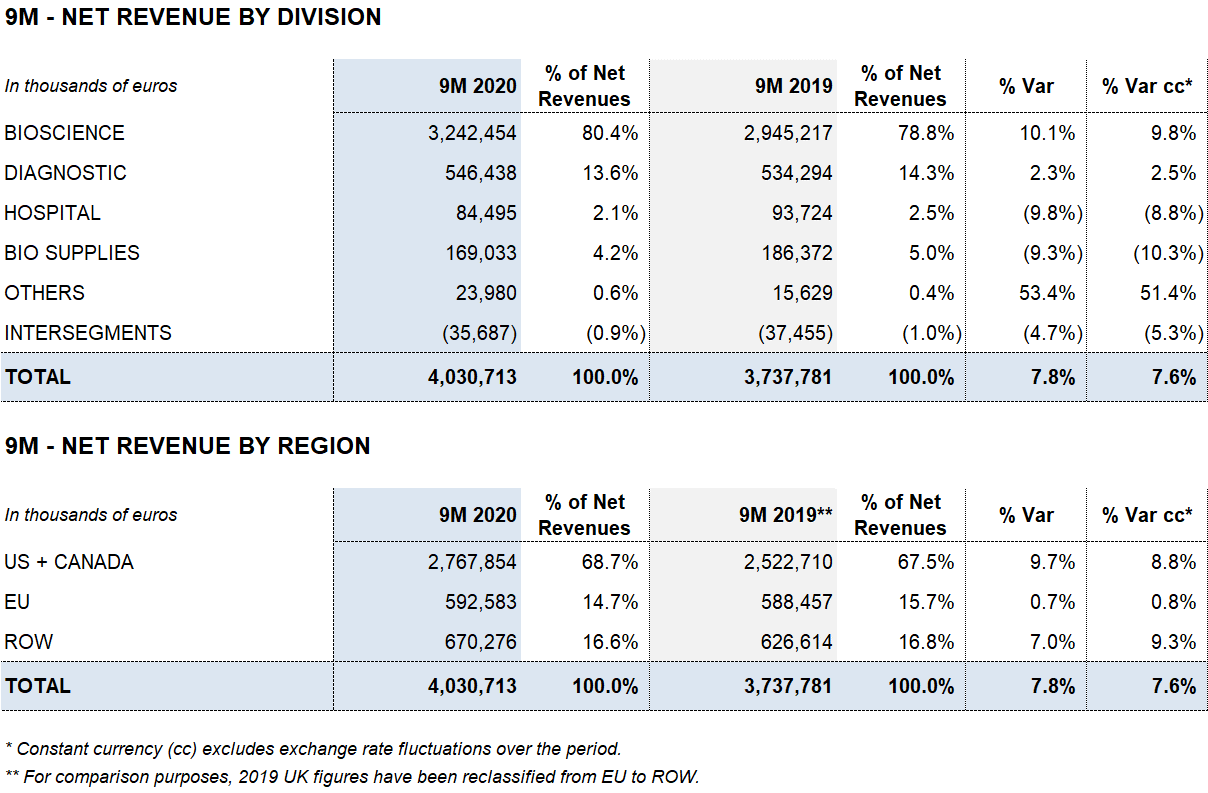
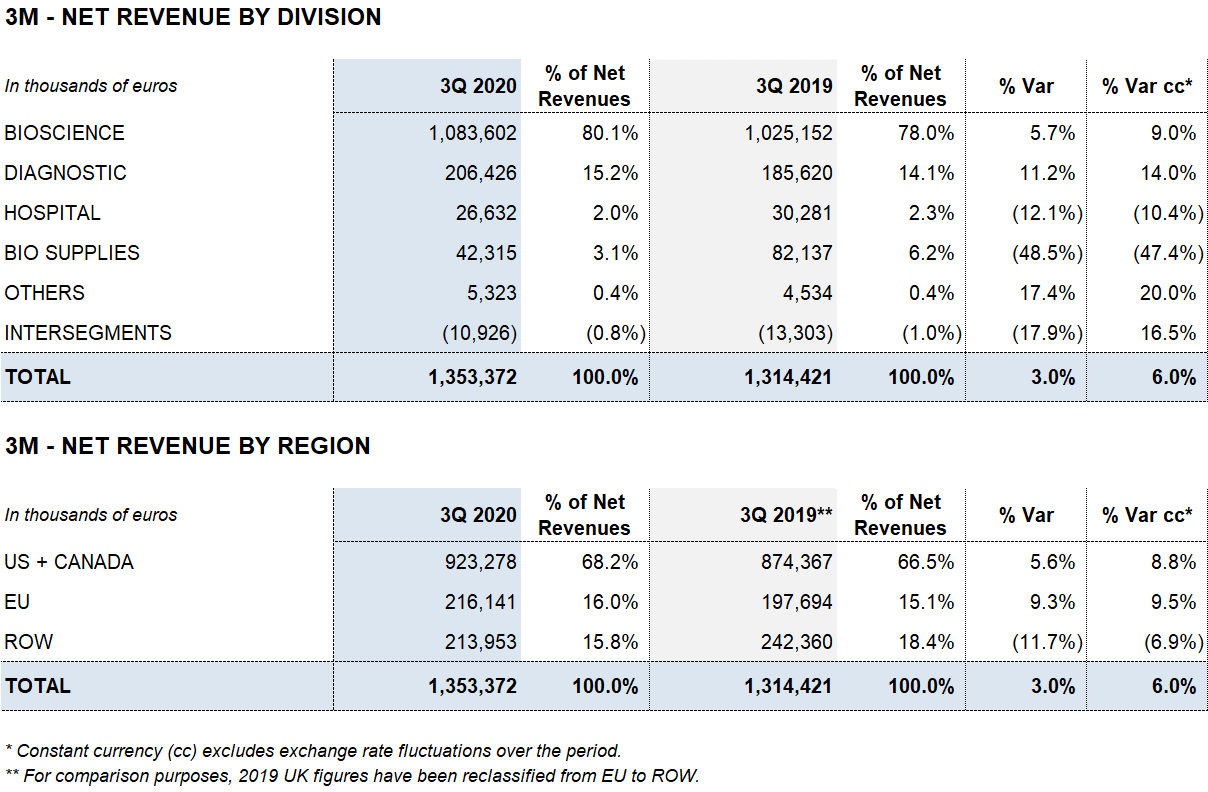
As of September 30, 2020, Grifols’ revenues increased by 7.8% (7.6% cc) to EUR 4,030.7 million, led by the robust performance of the Bioscience Division, growth in the Diagnostic Division and contributions from new products, which accounted for 35% of the company’s sales growth.
Bioscience Division sales increased by 10.1% (9.8% cc) to EUR 3,242,4 million. Third-quarter sales increased by 5.7% (9.0% cc) to EUR 1,083.6 million. This sustained growth was driven by robust sales of immunoglobulins in several markets, particularly the U.S. and Canada; albumin growth in China; and solid contributions from new products like Xembify®, VISTASEALTM and TAVLESSE®.
Diagnostic Division revenues also rose significantly in the third quarter of 2020, growing by 11.2% (14.0% cc) to EUR 206.4 million as a result of higher demand for TMA (Transcription-Mediated Amplification) molecular tests mainly in Spain, used to detect the SARS-CoV-2 virus. The division recorded a 2.3% (2.5% cc) increase in revenues, reaching 546.4 million in the first nine months of the year.
COVID-19 continues to impact the Hospital Division, with revenues of EUR 84.5 million, declining by 9.8% (8.8% cc).
Bio Supplies Division recorded EUR 169.0 million in sales, a 9.3% (10.3% cc) drop compared to the EUR 186.4 million reported in the same period last year. This overall decline is due mainly to the roll-off of certain contracts overseeing third-party plasma sales. As planned, this will enable Grifols to manage additional plasma volume to fuel growth of plasma-derived therapies. Excluding it, Bio Supplies Division revenues increased by 61.3% cc for the first nine months.
As part of its commitment to patients, Grifols remains committed to strengthening its plasma supply. In the U.S., an upward trend in plasma donations was observed in July, August and September. Meanwhile, Germany is showing a sooner recovery path than in the U.S. and it is expected to continue moving towards those levels reported in 2019 along second half of 2020.
With the implementation of its expansion plan, the company expects to increase its plasma supply by roughly 30% in 2021.
Amid the current global pandemic, Grifols believes more than ever in the potential of plasma and its overarching mission to meet the evolving needs of patients and society. In this regard, the company added its voice to the call made by the Protein Therapeutics Association (PPTA) during the 2020 International Plasma Awareness Week, urging global healthcare authorities to take decisive action to increase the volume of plasma donations in Europe.
At present, Grifols operates more than 310 plasma centers, which represent an important competitive advantage. The company continues its efforts to expand its network through both organic and inorganic growth. The recent acquisition of 11 U.S. plasma centers from South-Korea based Green Cross is one example of this forward-thinking strategy.
In June, Grifols booked an estimated impact of EUR 205 million for the entire 2020 fiscal year to adjust its inventory value primarily due to the COVID-19 pandemic.
Likewise, Grifols is making headway on its operating expense containment plan, expected to yield a positive impact of EUR 100 million in the 2020 profit and loss account. The plan will have no impact on Grifols’ labor force or innovation investments.
As of September 30, 2020, total operating expenses savings amounted to EUR 77 million. The company is working to make a significant part of the EUR 100 million plan permanent.
Reported EBITDA amounted to EUR 983.6 million, a margin of 24.4%, for the first nine months of 2020. The product mix and contribution from new high-margin products, in addition to the implementation of the cost-containment plan, elevated the company’s EBITDA in the third quarter to EUR 403.7 million, with a 29.8% margin.
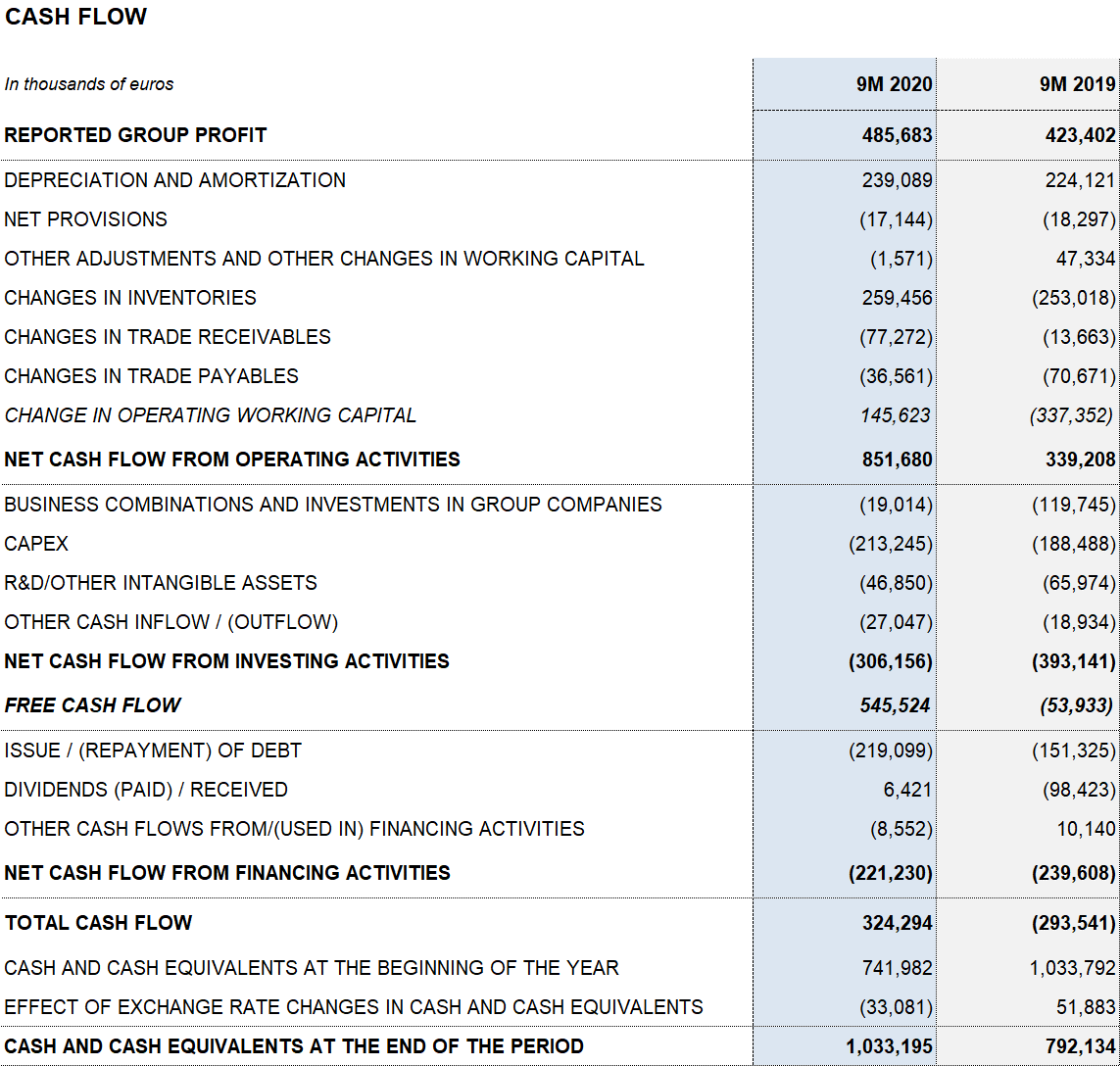
CAPEX levels totaled EUR 213.2 million (compared to EUR 188.5 million on September 30, 2019), while total net R+D+i investments amounted to EUR 247.3 million (EUR 244.6 million until September 2019), including in-house, external and investee-led projects. Both figures reinforce the company’s growth strategy, built on a sustainable, long-term and innovation-driven business model.
Within the framework of its firm commitment to society and innovation, Grifols leads more than 25 international initiatives dedicated to discovering potential COVID-19 treatments.
Among these, Grifols’ research to develop an anti-SARS-CoV-2 hyperimmune immunoglobulin using plasma from recovered COVID-19 donors is especially noteworthy. At present, the company is participating in an international clinical trial to assess the safety, tolerability and efficacy to treat COVID-19. This trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), which forms part of the U.S. National Institutes of Health (NIH).
The company is also making headway on a clinical trial to evaluate the efficacy of high-dosage intravenous immunoglobulin to stabilize or improve the health of COVID-19 patients. At the same time, it is collaborating in several European and U.S. studies with plasma-derived products, including antithrombin III and alpha-1 antitrypsin.
Grifols’ financial result was EUR 133.5 million for the first nine months of 2020 (EUR 265.4 million in the same period of 2019). This figure mainly includes the EUR 74.3 million reduction in net financial expenses following the debt refinancing process, closed in November 2019; and the positive EUR 56.5 million gain from the closing of the Shanghai RAAS transaction in the first quarter of 2020.
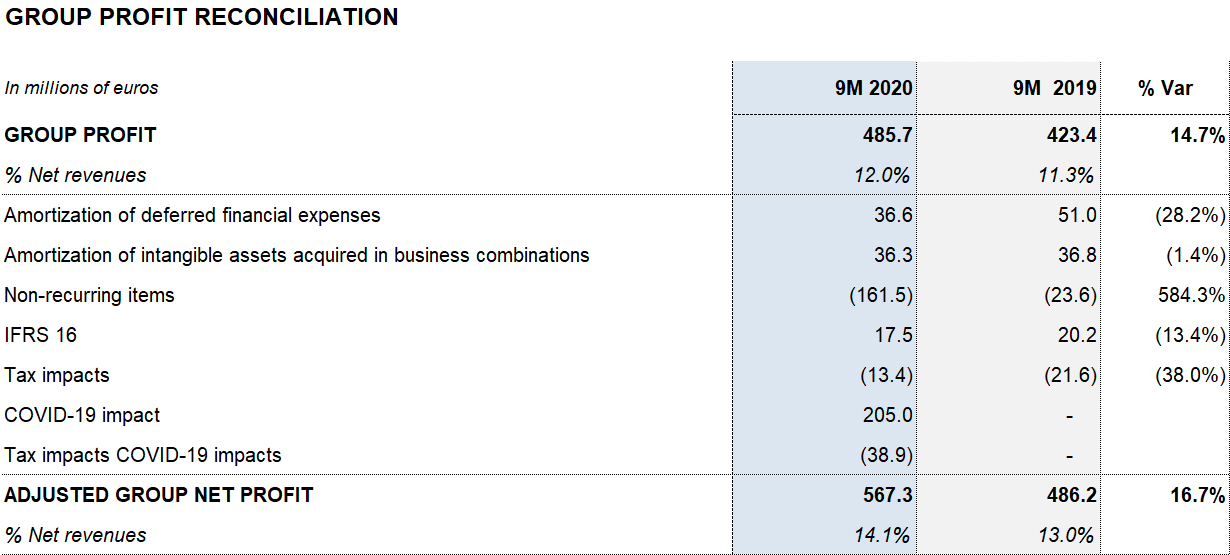
As of September 30, 2020, reported net profit totaled EUR 485.7 million (EUR 423.4 million in 2019), affected mainly by COVID-19 impacts, the operating-expense containment plan, stronger financial results and investee contributions, specifically the write-up of the equity stake in Alkahest (EUR 86.7 million) following the acquisition of the remaining equity capital.
As of September 30, adjusted net profit stands at EUR 567.3 million (EUR 486.2 million in 2019).
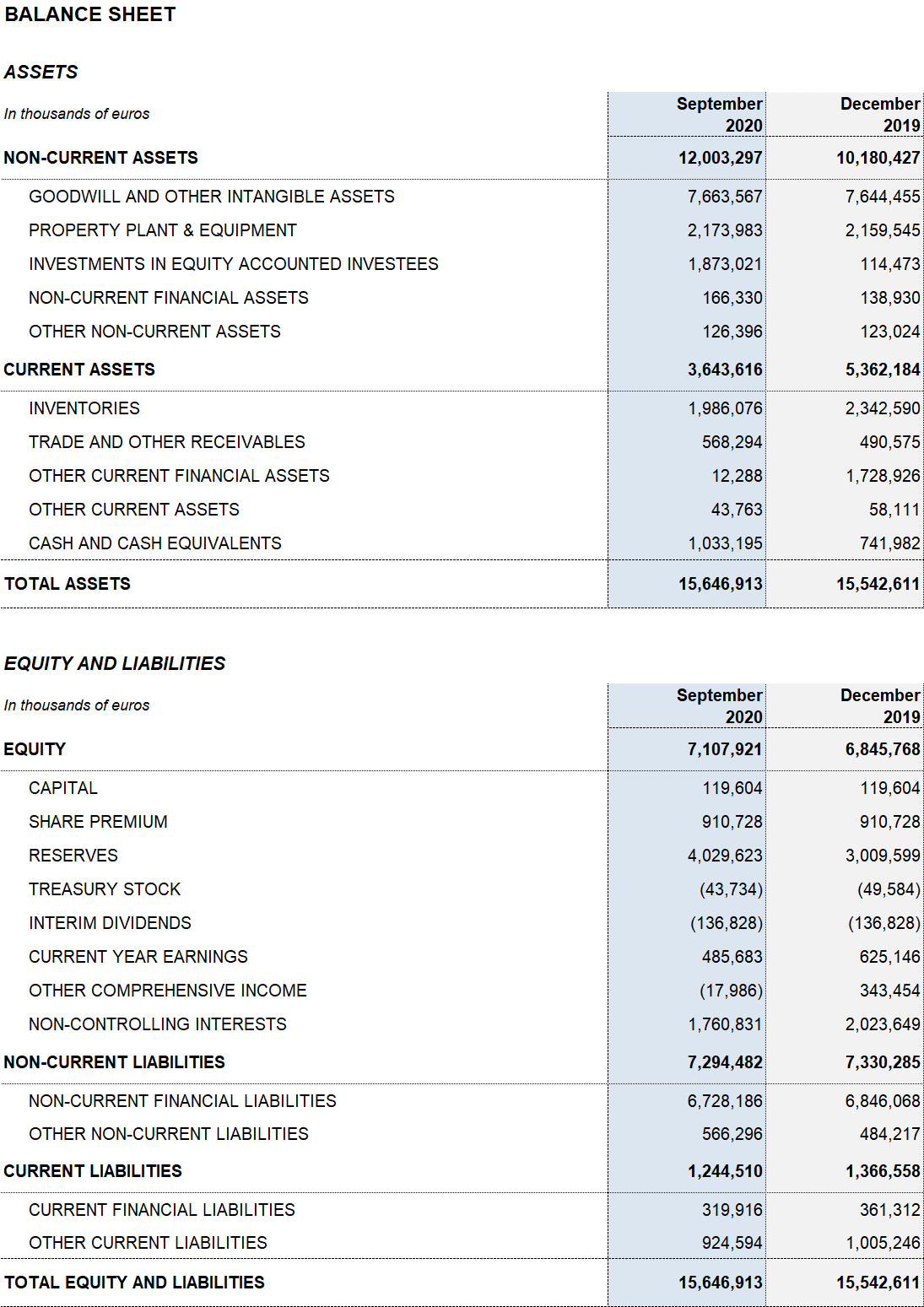
Excluding the impact of IFRS 162, the net financial debt totaled EUR 5,273.9 million. The net financial debt over EBITDA ratio improved to 4.1x.
Grifols upholds a robust liquidity position. As of September 30, 2020, its cash positions stand at EUR 1,033.2 million, which, when added to EUR 938 million in undrawn lines of credit, bring its liquidity position to approximately EUR 2,000 million.
Following the refinancing process completed in November 2019, the company does not face significant maturity repayments or down payments until 2025.
The company is prepared to respond to the demands of the current context and remains confident in its long-term growth strategy. The company will continue to closely monitor potential impacts on its operations and take all measures to mitigate them, if necessary.
PERFORMANCE BY DIVISION
Bioscience Division
The Bioscience Division continues to lead Grifols’ growth, achieving a 10.1% (9.8% cc) increase in revenues to EUR 3,242.4 million during the first nine months of the year.
The division’s sales grew by 5.7% (9.0% cc) to EUR 1.083.6 million in the third quarter, driven by the solid demand of the main plasma proteins, specifically immunoglobulin and albumin, as well as the positive evolution of new products like Xembify® and VISTASEALTM.
Demand for immunoglobulins remains very robust in high per-capita consumption markets, including the U.S., Canada and several countries in the European Union (EU) and Latin America. In order to adapt to patients’ specific needs, Grifols offers several immunoglobulins in both intravenous or subcutaneous administrations (Xembify®), with sales steadily rising in the United States.
Alpha-1 antitrypsin continues as one of the division’s core drivers, with particularly strong sales in countries such as the U.S. and Canada. Albumin sales continue to grow in China.
In terms of new product launches, of note are sales of Grifols’ biological sealant, developed and manufactured by the company as a surgical bleeding-control solution using a combination of two plasma proteins (fibrinogen and thrombin). Launched in the last quarter of 2019, the product is sold and distributed by Ethicon under the trade name VISTASEALTM.
The market launch of TAVLESSE® (fostamatinib) in certain European countries is also worth highlighting. Included within Grifols’ agreement with Rigel Pharmaceuticals, this product is used to treat chronic immune thrombocytopenia (ITP) in adult patients who are refractory to other treatments.
Diagnostic Division
The Diagnostic Division reported remarkably solid results in the third quarter, increasing by 11.2% (14.0% cc) to EUR 206.4 million. The division reported EUR 546.4 million in revenues in the first nine months of 2020, a 2.3% (2.5% cc) increase over the same period in 2019.
Especially notable was demand for the specialty diagnostic test to detect the SARS-CoV-2 virus mainly in Spain, leading to higher sales of Grifols’ NAT technology systems (Procleix® NAT Solutions), which incorporates Transcription Mediated Amplification (TMA). TMA is a commonly used technique thanks to its high sensitivity and capacity to automatically process large volumes of samples.
The blood-typing line also recorded notable growth in countries like China, while maintaining its upward trend in the U.S. This line includes both analyzers (Erytra®, Erytra-Eflexis® and Wadiana®) and reagents (DG-Gel® cards, red blood cells and anti-serums).
Hospital Division
The Hospital Division reported EUR 84.5 million in revenues, decreasing by 9.8% (8.8% cc) due to lower hospital investments as a result of the COVID-19 pandemic.
Pharmatech, intravenous solutions and medical devices were the main business lines impacted by this decline, which was partially offset by an increase in revenues from third-party manufacturing services.
Bio Supplies Division
The Bio Supplies Division totaled EUR 169.0 million in revenues during the first three quarters, decreasing by 9.3% (10.3% cc) compared to the same period in the previous year.
Third-party plasma sales declined in the third quarter as several third-party contracts rolled-off. This will enable Grifols to manage additional plasma volume in order to fuel growth of plasma-derived therapies.
Excluding third-party plasma sales, Bio Supplies Division revenues increased by 61.7% (61.3% cc) for the first nine months and 20.6% (23.1% cc) in the third quarter.
INNOVATION ACQUISITIONS
Grifols collaborates on a recently initiated clinical trial on the anti-SARS-CoV-2 hyperimmune immunoglobulin to treat COVID-19 patients
Grifols is participating in a newly initiated clinical trial to test the safety, tolerability and efficacy of an anti-SARS-CoV-2 hyperimmune immunoglobulin as potential treatment against COVID-19.
The National Institute of Allergy and Infectious Diseases (NIAID), which forms part of the U.S. National Institutes of Health, is sponsoring the trial.
This phase-3 study – called Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) – represents the first international multicenter trial on an anti-SARS-CoV-2 hyperimmune immunoglobulin. The trial is randomized, double-blind, placebo-controlled and adaptive, and comprises 500 hospitalized adult COVID-19 patients across 58 hospitals in 18 countries, including the U.S. and Spain.
Grifols is also leading more than 25 research initiatives on potential therapies to treat different phases of the disease.
Acquisition agreement with Alkahest to enhance R+D
In March 2015, Grifols acquired a 45% stake in Alkahest. Following a five-year collaboration, Grifols has reached an agreement to buy the remaining shares for USD 146 million on a debt-free basis.
Alkahest’s research on plasma-based and non-plasma therapies will enable Grifols to diversify its research lines while maintaining its core focus on plasma science.
Alkahest currently has four candidates in six phase-2 clinical trials, including therapeutic products for neurodegenerative disorders, cognitive decline, and neuromuscular and ophthalmic diseases.
Alkahest has developed a map of the human plasma proteome, facilitating the identification of plasma proteins and their recombinant analogs as potential medicines.
Grifols closes the acquisition of manufacturing facilities in Canada and 11 plasma centers in the U.S.
As announced in July, Grifols closed an agreement with the South Korean firm, GC Pharma (Group) to acquire a fractionation plant and two purification plants (one for immunoglobulin and another for albumin) in Montreal (Canada) for USD 370 million, and, in a separate transaction, 11 plasma centers in the U.S., property of Green Cross, for USD 90 million.
This transaction aligns with Grifols’ international sustainable growth strategy, which aims to increase the company’s supply of plasma and reinforce its global expansion among its core objectives.
Through this strategic acquisition, Grifols will become the only large-scale commercial manufacturer of plasma products in Canada. At the same time, it will enhance Grifols’ international expansion by strengthening its presence in North America, since the Canadian market has one of the world’s highest rates of plasma consumption per capita and significant growth potential.
The operation also supports Canada’s objective of achieving greater self-sufficiency in plasma-derived products, which has become especially critical in light of the current pandemic.
At present, Grifols operates a broad network of more than 310 plasma centers in the U.S. and Europe, which has enabled the company to expand and diversify its access to plasma.
ADDITIONAL INFORMATION
Annual Investors and Analysts Meeting
Despite the current pandemic and mobility restrictions, Grifols held its annual meeting with investors and analysts in October via a telematic platform.
The meeting covered core topics such as Grifols’ integrated innovation strategy, including its initiatives to combat COVID-19 and its Alkahest projects; commercial strategies, offering a holistic vision on its operations and strategic alliance in China; plasma supply and industrial capacities; and the company’s financial and non-financial performance.
Grifols’ co-CEOs and several members of the senior management participated in the event.
Grifols General Shareholders Meeting
Grifols celebrated its Annual General Shareholders Meeting in an exclusively telematic format, with nearly 74% of the share capital represented.
All of the agenda items were endorsed, including the decision by Grifols’ shareholders to distribute more than EUR 250 million to dividends (approximately EUR 0.36 gross per share) in 2019.
Over the last five years, Grifols has paid more than EUR 1,200 million to dividends, evidence of its firm commitment to generate value for shareholders.
1Operational or constant currency (cc) excludes the impact of exchange rate variations during the period.
2As of September 30, 2020, the impact of IFRS 16 on total debt was EUR 741.0 million.