Barcelona (Spain), December 6, 2019- Grifols (MCE: GRF, MCE: GRF.P, NASDAQ: GRFS), a leading global producer of plasma-derived medicines, today concluded more than a year of encouraging results of its AMBAR (Alzheimer Management by Albumin Replacement) clinical trial with the presentation of new neuroimaging data that show the reduction in the progression of the disease in patients with mild-to-moderate Alzheimer’s disease.
These findings, released at the Clinical Trials on Alzheimer’s Disease (CTAD) Conference 2019 in San Diego, Calif. (USA), strengthen Grifols’ investigative approach using Plasma Protein Replacement Therapies.
The neuroimaging, which was measured by the FDG-PET1 technique, shows positive results particularly in patients receiving both albumin and immunoglobulin (IG). In comparison with the placebo group, these patients had less reduction of brain glucose metabolism over the 14 months of the clinical trial. This suggests that neuronal damage was reduced in these patients (see figure).
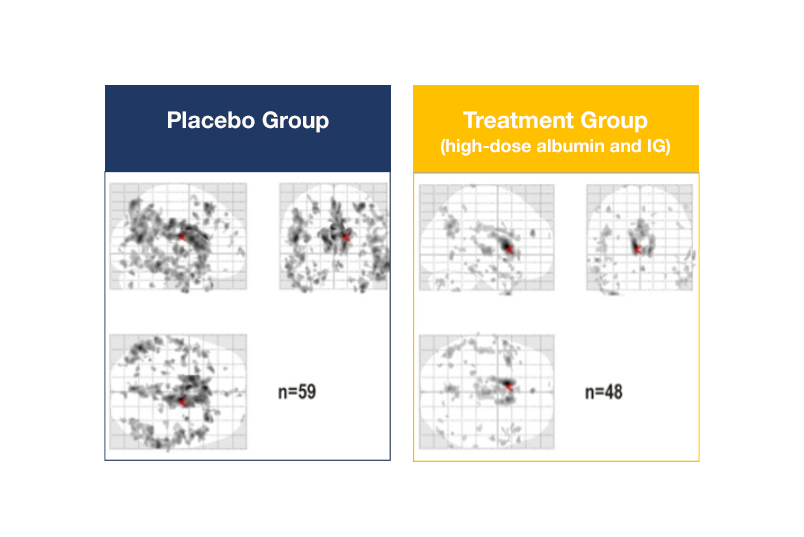
Changes in brain glucose metabolism in patients treated with high-dose albumin and immunoglobulin (IG) [right] versus placebo [left].
The images display the extension and distribution of the dark spots that relate to the reduction of glucose metabolism in the brain. Fewer and lighter spots suggest less neuronal damage, which is observed in the patients receiving both albumin and IG compared with placebo.
Grifols presented the first AMBAR results at the 11th CTAD Conference in Barcelona in October 2018. The primary efficacy endpoints, the ADAS-Cog2 and ADCS-ADL3 scales, showed a 61% reduction in disease progression for both measures in the cohort of patients with moderate Alzheimer’s.
Additional data covering the secondary endpoints of the study, such as memory and language, were presented at the 14th International Conference on Alzheimer's and Parkinson's Diseases (AD/PD) in Lisbon (Portugal) in March 2019, and showed a positive impact in both mild and moderate patients.
Results of other relevant endpoints, CDR-Sb4 and ADCS-CGIC5, evaluating both functional and cognitive capacity, were presented at the Alzheimer’s Association International Conference (AAIC) 2019 in Los Angeles, Calif. (USA) in July, and also pointed in the same direction when considering all treated patients together.
The CDR-Sb scale – which assesses memory, orientation, judgment, community affairs, home and hobbies, and personal care – showed a 71% reduction in clinical decline with respect to placebo in patients treated as a whole and in all three clinical trial treatment arms analyzed separately.
For the ADCS-CGIC scale – which assesses several domains of cognition, daily functioning and behavior from both the patient and the caregiver perspective – a stabilization was observed in all treated patients with respect to placebo. This effect remained in all three clinical trial treatment arms when analyzed separately.
“The AMBAR trial’s results are encouraging news for mild-to-moderate Alzheimer’s patients. These findings are the consequence of 15 years of rigorous scientific research at Grifols and the company will continue investigating this devastating condition that affects millions of patients around the world,” said Dr. Antonio Páez, Medical Director of the AMBAR Clinical Program at Grifols.
Grifols will soon meet with the FDA to discuss the AMBAR clinical development program and the design of a successive AMBAR II trial that will deepen and complement the just-concluded one.
1FDG-PET: Fluorodeoxyglucose Positron Emission Tomography.
2ADAS-Cog: Alzheimer’s Disease Assessment Scale – Cognitive.
3ADCS-ADL: Alzheimer’s Disease Cooperative Study – Activities of Daily Living.
4CDR-Sb: Clinical Dementia Rating Scale – Sum of Boxes.
5ADCS-CGIC: Alzheimer’s Disease Cooperative Study – Clinical Global Impression of Change.
About AMBAR
AMBAR is an international, multicenter, randomized, double-blind, placebo-controlled, with parallel assignment clinical trial that enrolled patients with mild and moderate Alzheimer’s from 41 treatment centers in Spain and the United States. The study was designed to evaluate the efficacy and safety of short-term plasma exchange followed by long-term plasmapheresis with infusion of albumin combined with intravenous immunoglobulin in patients with mild and moderate Alzheimer’s disease.
AMBAR was designed to evaluate whether the progression of Alzheimer's could be stabilized through therapeutic plasma exchange, a process that entails periodically extracting plasma and infusing albumin solution (Albutein®) with or without intravenous immunoglobulin (Flebogamma® DIF).
AMBAR targets a multimodal approach to the management of the disease based on the hypothesis that most of the amyloid-beta protein – one of the proteins accumulated in the brains of Alzheimer's patients – is bound to albumin and circulates in plasma. Extracting this plasma may flush amyloid-beta peptide from the brain into the plasma, thus limiting the disease's impact on the patient's cognitive functions. Additionally, albumin has binding capacity and antioxidant properties, and both albumin and immunoglobulin display immunomodulatory and anti-inflammatory properties.
The AMBAR study included 496 mild and moderate Alzheimer’s patients, 55-85 years-old, randomized in three treatment groups and one control (placebo) group. An independent contract research organization (CRO) oversaw the trial's clinical monitoring phase and managed the data collection and analysis stages. The trial employed a randomized and double-blind design, meaning that neither patients nor evaluators knew whether subjects were receiving the treatment or the placebo.
The company began its research on Alzheimer's disease in 2004 with several preclinical trials, two pilot studies and a Phase II clinical trial before launching the AMBAR trial.
Fundació ACE in Barcelona, Spain, and the Alzheimer Research Center of the University of Pittsburgh, PA, USA, have been instrumental partners in the AMBAR research and in Grifols Alzheimer’s program since its initiation in 2004.
For more information on the AMBAR study and the results presented visit: ambar.grifols.com
