Our commitment to patients and research
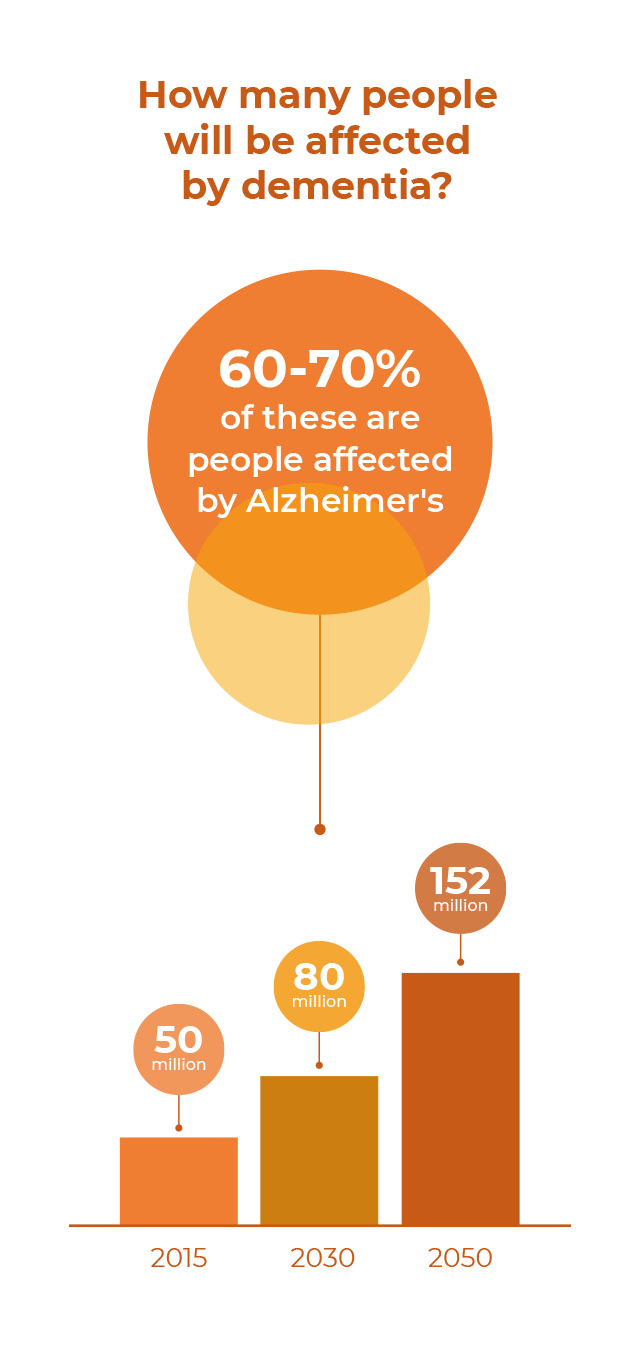
Currently around 55 million people are living with Alzheimer’s disease and other devastating dementias, a number projected to jump to 152 million by the middle of the century, a result of a graying global population as well as more and better diagnoses.
Alzheimer's disease is the most common form of dementia and accounts for as many as 70% of all cases. Its prevalence has made it one of the most pressing healthcare challenges facing society. The exact cause of the disease, which leads to a decline in memory, reasoning and the ability to perform routine daily activities, is not yet fully understood, though it’s believed to be multifactorial. While treatments are available to alleviate its symptoms, there is currently no cure.
Source: WHO (www.who.int)
©2021 Grifols, S.A. All rights reserved worldwide.
Alzheimer’s ravages people and takes an incalculable emotional and financial toll on their loved ones and caregivers. The disease is also a burden on governments and healthcare systems.
As part of its commitment to diagnosing and treating Alzheimer’s, Grifols has invested more than €200 million since 2004 and will continue to dedicate its best and brightest minds to finding solutions for this global public health crisis.
Over 35 million
people worldwide
live with Alzheimer's disease
Nearly 20
years of research
on Alzheimer's
€200+
million invested in
Alzheimer's research since 2004
Our integrated strategy for this neurodegenerative disease features AMBAR® – Alzheimer Management by Albumin Replacement – a multimodal approach designed to slow the disease’s progression. It consists of two vitally linked steps. First, therapeutic plasma exchange (TPE) flushes out pathological substances such as amyloid-beta, a protein that binds to the plasma protein albumin and whose accumulation in the brain is believed to contribute to Alzheimer’s.
Then fresh albumin, capable of capturing more amyloid beta, is infused to repeat the restorative process. Patients also can potentially benefit from albumin’s antioxidant capacity and immunomodulatory properties.
In our pioneering AMBAR® study, the primary efficacy endpoints, the ADAS-Cog* and ADCS-ADL scales,** showed a 61% reduction in disease progression for both measures in a prespecified subgroup analysis of the cohort of patients with moderate Alzheimer’s, although the ADAS-Cog showed a borderline statistical significance (p=0.05). Secondary endpoints showed a positive impact in patients with both mild and moderate Alzheimer’s.
Grifols, committed to advancing the AMBAR® clinical program, is also exploring new lines of Alzheimer's research through our subsidiaries Alkahest and Araclon.
*ADAS-Cog: Alzheimer’s Disease Assessment Scale – Cognitive.
**ADCS-ADL: Alzheimer’s Disease Cooperative Study – Activities of Daily Living.
AMBAR® Procedure
AMBAR® entails a multimodal approach to the management of Alzheimer's. Periodic plasma exchange flushes out amyloid-beta protein, which binds to albumin and whose accumulation in the brain causes neuronal damage. Then fresh albumin infusions, in addition to eliminating more amyloid-beta through TPE, may also have other beneficial effects due to its antioxidant and anti-inflammatory properties.